
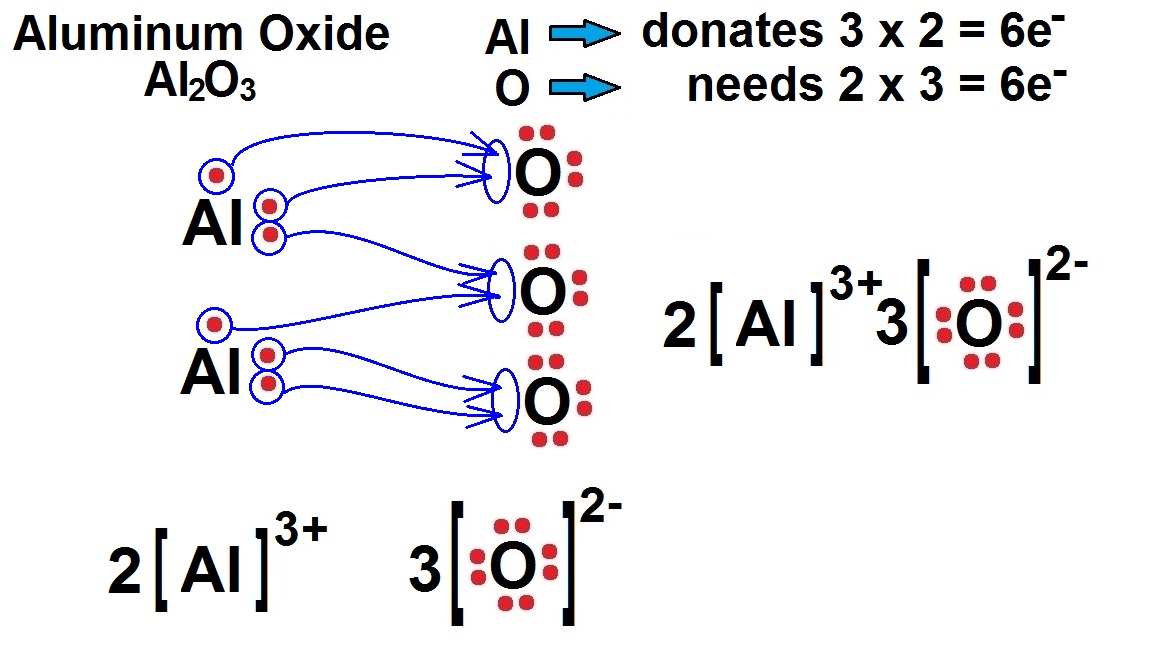
Explain The Formation Of Ionic Compound Al2o3 With Electron Dot Structure Chemistry Metals
It is the combination of metal and non-metal compounds. Thus it forms an ionic compound. Al2O3 is composed of 2 aluminum metals and 3 oxygen atoms. Both Al atoms lost their 3 valence electrons to 3 O atoms thus it has a +3 charge. The 3 O atoms gain 2 electrons from 2 Al atoms thus it has a -2 charge on it.

Different structures of (Al2O3)2 (Al and O atoms are depicted by red... Download Scientific
Contents show Lewis Structure of Al2O3 The concept of Lewis structure was first introduced by Gilbert N. Lewis in 1916. It is also known as the Lewis dot diagram or electron dot structure. It is the structural illustration of the position of the valence electrons, involved in the formation of a chemical bond, around the atoms inside a molecule.

Aluminum Chloride Electron Dot Diagram For Aluminum Chloride
We can determine the Lewis structure as follows: Aluminum (Al) has 3 valence electrons, and oxygen (O) has 6 valence electrons. Aluminum wants to lose 3 electrons to achieve a stable configuration, and oxygen wants to gain 2 electrons.

How to Draw the Lewis Dot Structure for Al2O3 Aluminum oxide YouTube
The structure is an intermediate between α-Al2O3 and γ-Al(OH)3, a fully hydroxylated form of alumina. A semiordered oxygen layer about 2.3 angstroms above the terminal oxygen layer is interpreted as adsorbed water. The clean α-Al2O3 (0001) surface, in contrast, is Al terminated and significantly relaxed relative to the bulk structure.

CLASS 9TH SCIENCE TABLE 3.6 POLYATOMIC ION AL2O3 LEWIS DOT STRUCTURE. YouTube
Science Chemistry Chemistry questions and answers Write the Lewis structure for Al_2O_3. Draw the Lewis dot structure for Al_2O_3. Include all lone pairs of electrons. This problem has been solved! You'll get a detailed solution from a subject matter expert that helps you learn core concepts. See Answer

determine the lewis dot structure of al2o3 Brainly.in
A, 26, 380, (2008). Properties of Al2O3 Aluminum oxide can possess several different structures. As the compound is annealed for longer periods of time and at higher temperatures, it changes structure, which ultimately influences the properties of the compound. The transition of Al2O3 with heat:

Al2o3 Aluminum Oxide Lewis Electron Dot Structure Model Blog PNG Image Transparent PNG Free
The aluminum oxide (Al 2 O 3) or alumina, which is largely applied in several arenas such as electronic, ceramics, high thermal material and catalysts etc.The current work was design with aluminum oxide quantum dots (γ-Al 2 O 3 QDs) and plays a crucial role for the concentration based analysis of hydrogen peroxide (H 2 O 2, referred to as HP).The γ-Al 2 O 3 was synthesized via hydrothermal.

Is CS2 (Carbon disulfide) Ionic or Covalent/Molecular? YouTube
Visit http://ilectureonline.com for more math and science lectures!In this video I will show the Lewis structure for ionic compound for aluminum oxide, Al2O3.
14+ Cacl2 Lewis Structure Robhosking Diagram
Lewis Structure of Al2O3, Aluminum Oxide chemistNATE 259K subscribers Subscribe 47K views 2 years ago Lewis Structures TWO Aluminum atoms, metals with 3 electrons in its outer shell, requires.

Aluminium oxide Al₂O₃ Molecular Geometry Hybridization Molecular Weight Molecular
This widget gets the Lewis structure of chemical compounds. Send feedback | Visit Wolfram|Alpha Get the free "Lewis Structure Finder" widget for your website, blog, Wordpress, Blogger, or iGoogle. Find more Chemistry widgets in Wolfram|Alpha.
The detailed geometric and electronic insights into the interface structure and evolution expand our understanding of this fundamental interface and have important implications for the engineering and design of Al /Al-based corrosion coatings with enhanced barrier properties, controllable transistor technologies, and noise-free superconducting q.

explain the formation of ionic compound al2o3 with electron dot structure. (given. atomic no. of
Lewis Structure of Al2O3, Aluminum Oxide Aluminium oxide is a solid ionic compound, made from atoms of one metal (Aluminum) that have lost three electrons each to become +3 cations, and atoms of a non-metal (oxygen) which have gained two electrons each to become -2 anions. The numbers 3 and 2 have a lowest common multiple of 6.

draw electron dot structure of aluminium oxide Brainly.in
Learn the electron dot structure of Al2O3 in the easiest way. You can also watch the complete video on electron dot structure of other compounds herehttps://.
Dwayne's Education Blog Dwayne Schnell's ePortfolio
Lewis Dot Structure for Al2O3 (Aluminum Oxide) Geometry of Molecules 3.28K subscribers Subscribe Subscribed 11 Share 1.1K views 1 year ago Lewis Structure Hello there! Today in this video.

Lewis Structure of Al2O3, Aluminum Oxide YouTube
25778 169720 63648 Submitted by Materials Project User remarks: High pressure experimental phase Aluminium oxide - Cr-doped Corundum Displaying lattice parameters for primitive cell; note that calculated cell volumes are typically overestimated on average by 3% (+/- 6%).

Electron Dot Diagram For Al
Aluminum oxide (Al2O3) is a compound composed of two aluminum (Al) atoms and three oxygen (O) atoms. To determine the Lewis dot diagram for Al2O3, we need to know the number of valence electrons for each element. Aluminum has three valence electrons, while oxygen has six valence electrons.